Bio-Rad SingleShot™ Cell Lysis RT-qPCR Kits User Manual
Page 6
© 2014 Bio-Rad Laboratories, Inc.
10042477
SingleShot
™
Cell Lysis Kit
Using the PrimePCR Reverse Transcription Control Assay to Determine Optimal Input
Lysate Volume
1. Resuspend the RNA control template in 200 µl of nuclease-free TE buffer pH 7.5.
2. Prepare cell lysate from either adherent (see Processing of Adherent Cells in a
96-Well Culture Plate section) or suspension cells (see Processing of Nonadherent
Cells in a 96-Well PCR Plate section) with an optimal number of input cells.
3. Vary input lysate in the RT-qPCR reactions as shown in Table 6 and Table 7.
4. Program the thermal cycling protocol on a real-time PCR instrument according
to manufacturer's instructions.
5. Use 2 µl of completed RT reaction and 1 µl of the PrimePCR Reverse Transcription
Control Assay in a 20 µl qPCR reaction.
Table 6. Setting up lysate titrations for two-step RT-qPCR reactions.
Table 7. Setting up lysate titrations for one-step RT-qPCR reactions.
Two-Step RT-qPCR
Input Lysate, %
Lysate Volume, µl
RNA Control
Template, μl
2x RT
Master Mix, μl*
Nuclease-Free
H
2
O, μl
10
2
1
10
7
20
4
1
10
5
30
6
1
10
3
40
8
1
10
1
45
9
1
10
0
One-Step RT-qPCR
Input Lysate, %
Lysate
Volume, µl
RNA Control
Template, μl
RNA Control
Assay, μl
2x One-Step
RT-qPCR Mix, μl
Nuclease-Free
H
2
O, μl
10
2
1
1
10
6
15
3
1
1
10
5
20
4
1
1
10
4
* Includes 5x iScript advanced reaction mix, iScript advanced reverse transcriptase, and nuclease-free H
2
O.
Cq
40
35
30
25
20
15
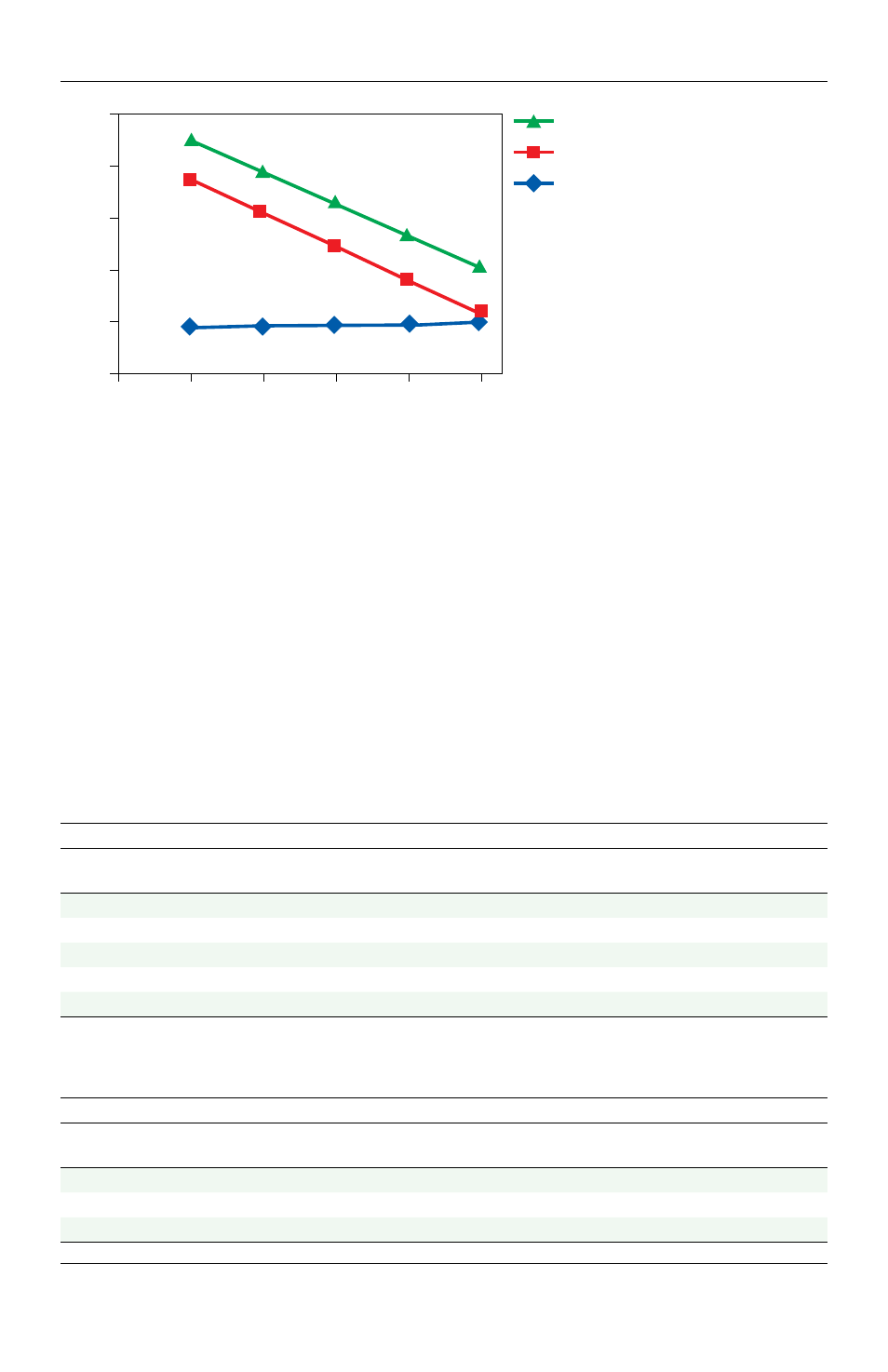
0 1 2 3 4 5
Cell number, log
Target 1
Target 2
Control RNA
Fig. 1. Determining optimal cell
input number. In this example,
10
5
input cells is the maximum input.
No inhibition was noted across the
input series. Target genes demonstrate
linearity across all four logs.
