Myron L ARH1 User Manual
Page 13
varies with concentration and temperature in a non-linear fashion. Other
solutions have more variation still. The ARH1 uses corrections that
change with concentration and temperature instead of single average
values (see Chart 1, pg. 19).
C. An Example of 2 different solution selections and the
resulting compensation:
How much error results from treating natural water as if it were KCl at
15°C?
A tap water solution should be compensated as 442 with a tempco of
1.68 %/°C, where the KCl value used would be 1.90 %/°C.
Suppose a measurement at 15°C (or 59°F) is 900 microsiemens of true
uncompensated conductivity.
Using a 442 correction of 10 (degrees below 25) x 1.68% indicates the
solution is reading 16.8% low. For correction, dividing by (.832) yields
1082 microsiemens as a compensated reading.
A KCl correction of 10 (degrees below 25) x 1.9% indicates the solution
is reading 19% low. Dividing by (.81) yields 1111 microsiemens for a
compensated reading. The difference is 29 out of 1082 = 2.7%.
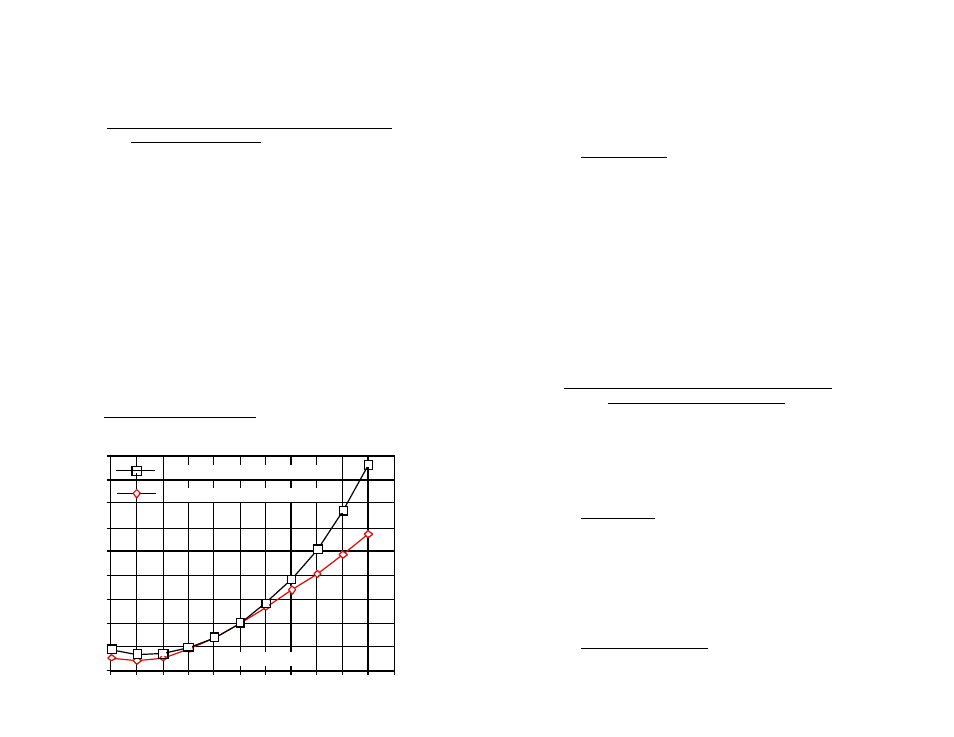
D. A Chart of Comparative Error
In the range of 1000 µS, the error using KCl on a solution that should be
compensated as NaCl or as 442, is shown in the graph below.
Users wanting to measure natural water based solutions to 1% would
have to alter the internal compensation to the more suitable preloaded
“442” values or stay close to 25°C. Some who have standardized to KCl
based compensation may want to stick with it, regardless of increasing
error as you get further from 25°C. The ARH1 will provide the repeatability
and convertibility of data needed for relative values for process control.
E. Other Solutions
A salt solution like sea water or liquid fertilizer acts like NaCl. An internal
correction for NaCl can be selected for greatest accuracy with such
solutions. Many solutions are not at all similar to KCl, NaCl or 442. A sugar
solution, or a silicate, or a calcium salt at a high or low temperature may
require a value peculiar to the application to provide readings close to the
true compensated conductivity.
Clearly, the solution characteristics should be chosen to truly represent
the actual water under test for rated accuracy of ±1% of full scale. Many
industrial applications have always been relative measurements seeking a
number to indicate a certain setpoint or minimum concentration or trend.
The ARH1 gives the user the capability to take data in “KCl conductivity
units” to compare to older published data, as in terms of NaCl or 442, or
as may be appropriate.
XIII.
CONDUCTIVITY CONVERSION to TOTAL
DISSOLVED SOLIDS (TDS)
Electrical conductivity indicates solution concentration and ionization of
the dissolved material. Since temperature greatly affects ionization,
conductivity measurements are temperature dependent and are normally
corrected to read what they would be at 25°C (ref. Temperature
Compensation, pg. 19).
A. How it’s Done
Once the effect of temperature is removed, the compensated
conductivity is a function of the concentration (TDS). Temperature
compensation of the conductivity of a solution is performed automatically
by the internal processor, using data derived from chemical tables. Any
dissolved salt at a known temperature has a known ratio of conductivity to
concentration. Tables of conversion ratios referenced to 25°C have been
published by chemists for decades.
B. Solution Characteristics
Real world applications have to measure a wide range of materials and
mixtures of electrolyte solutions. To solve this problem, industrial users
commonly use the characteristics of a standard material as a model for
20
21
Chart 2
(2)%
(1)%
0%
1%
2%
3%
4%
5%
6%
7%
0
5
10 15 20 25 30 35 40 45 50 55
Temperature
NaCl error with KCl tempco
442 error with KCl tempco
