Symbols – Widex BB4 User Manual
Page 55
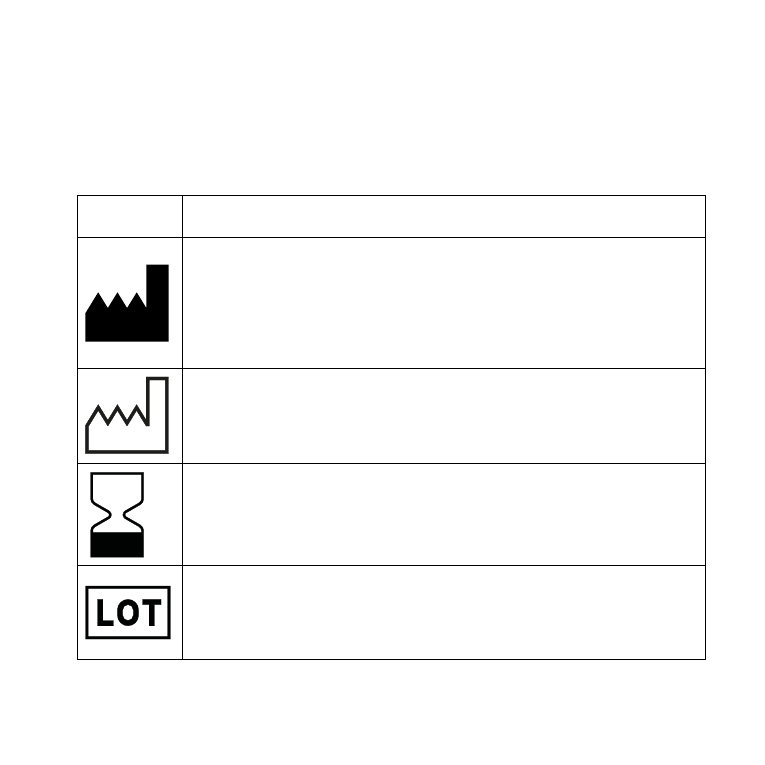
SYMBOLS
Symbols commonly used by Widex A/S in medical device labelling
(labels/IFU/etc .)
Symbol
Title/Description
Manufacturer
The product is produced by the manufacturer whose name
and address are stated next to the symbol . If appropriate,
the date of manufacture may also be stated .
Date of manufacture
The date when the product was manufactured .
Use-by date
The date after which the product is not to be used .
Batch code
The product’s batch code (lot or batch identification) .
55
