Ammonia distillation with the microblock – Environmental Express MicroBloc User Manual
Page 15
800.745.8218 / 843.576.1147 / www.environmentalexpress.com
Environmental Express
• 13
HotBlock:
Operation and Instruction Manual
MicroBlock
TM
Distillations
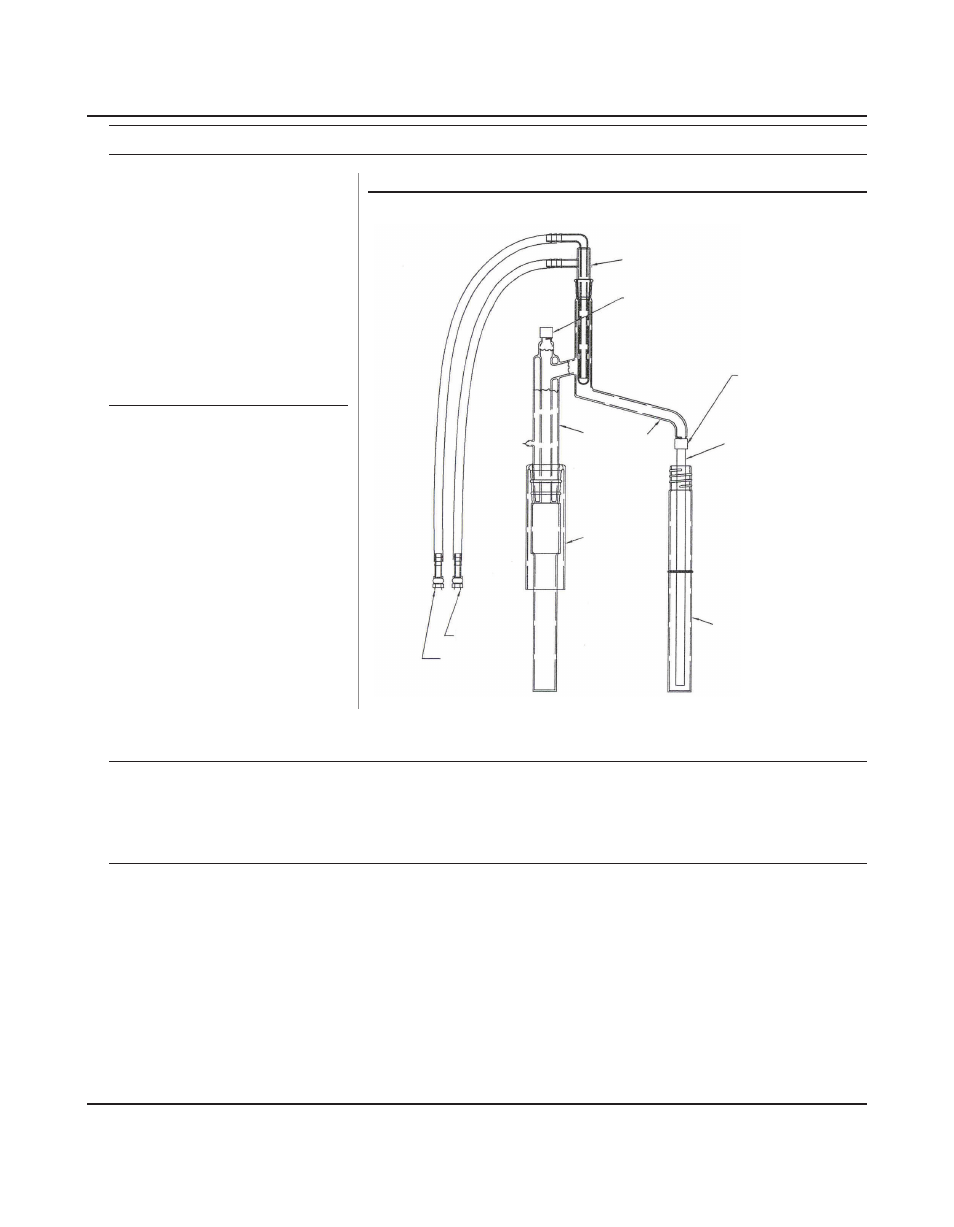
Ammonia Distillation with the MICROBLOCK
TM
Cold Finger
C5210
Cap C5213, and
PTFE-Faced Septa
C5218
Cap C5213,
and Silicone
Sealing Ring
C5225
Long Stem
Outlet Tube
for Ammonia
C5226
Receiver Tube
C5228
Distillation
Head
C5219
Reaction
Tube
C5214-B
Drain
Water Inlet
Figure 3 - Ammonia Glassware
1.0 SCOPE AND APPLICATION
1.1 This method covers the
determination of ammonia
in drinking, ground, surface,
and saline waters and
domestic and industrial
wastes.
1.2 The applicable range is 0.01
to 2.0mg/L ammonia as N.
Higher concentrations can be
determined by dilution.
2.0 SUMMARY OF METHOD
2.1 The sample is buffered at pH
9.5 with borate buffer for
aqueous samples to decrease
hydrolysis of cyanates and
other organic nitrogen
compounds. Under acidic
conditions these compounds
can form ammonia. The
sample is distilled into 0.04N
sulfuric acid or 2% boric
acid depending upon the
analytical technique used to
quantitate for ammonia.
2.2 Reduced volume versions of
this method that use the
same reagents and molar
ratios are acceptable provided they meet the quality control and performance requirements stated in the method.
3.0 INTERFERENCES
3.1 Cyanate in certain industrial wastes will hydrolyze to some extent even at pH 9.5, which is recommended for
distillation.
3.2 Residual chlorine must be removed prior to distillation by treatment with sodium thiosulfate or sodium sulfite.
4.0 CHEMICALS REQUIRED – DISTILLATION ONLY
Note: The toxicities for each of the reagents used in this method are not fully documented. Treat each chemical as a potential
health hazard and limit exposure. Exercise good laboratory technique with emphasis on safety.
4.1 Sodium Hydroxide
4.2 Sodium Tetraborate
4.3 Sulfuric Acid
4.4 Sodium Thiosulfate
4.5 Sodium Sulfite
4.6 Ammonium Chloride
