3B Scientific Pressure Container for Determining Weight of Air User Manual
Page 2
Elwe Didactic GmbH ▪ Steinfelsstr. 6 ▪ 08248 Klingenthal ▪ Germany ▪
www.elwedidactic.com
3B Scientific GmbH ▪ Rudorffweg 8 ▪ 21031 Hamburg ▪ Germany ▪
www.3bscientific.com
Rights to amend technical specification reservedn
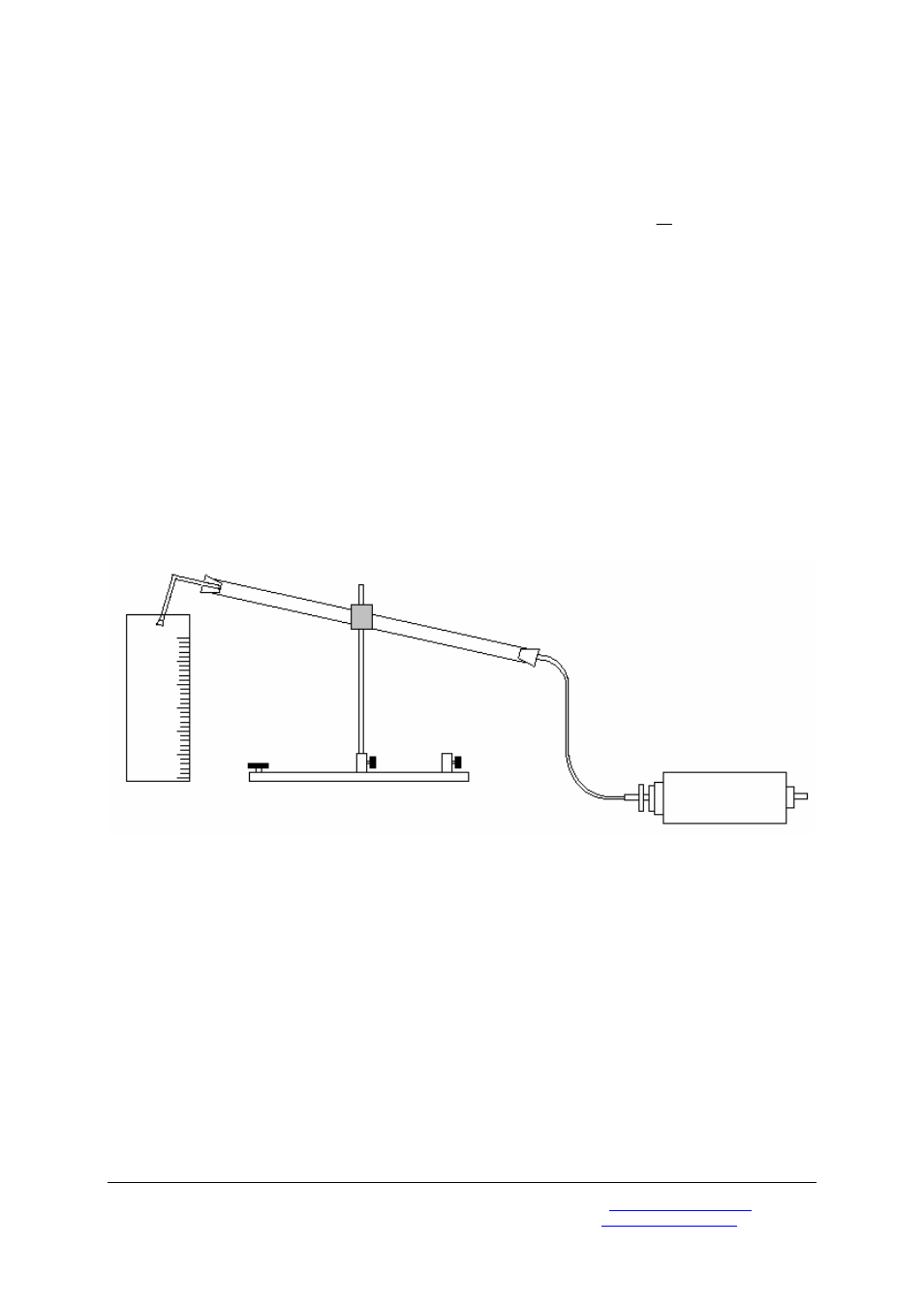
• Completely fill the glass tube with water.
• Close the end of the right-angled tubing
using a stopper with no hole in it.
• Clamp the glass tube to the stand at a
slight angle and adjust the height so that
the tube points into a measuring cylinder
placed beneath it.
• Take the bung out of the right-angle tube
and let any excess water drain out of it so
that the water level only comes up as far
as the bend. Empty this water out of the
measuring cylinder.
• Slowly open the outlet valve until all the
air has escaped from the capsule.
• The escaping air forces water out of the
glass tube. You should collect this water
in the measuring cylinder and measure its
volume.
The water so collected has the same volume
V as the air that has escaped from the cap-
sule.
• Use the values you have obtained to cal-
culate the density of air using the formula
V
m
=
ρ
Perform the experiment several timesto
obtain an average value for ρ.
• From the value of ρ you have measured,
make corrections to extrapolate its value
for standard atmospheric conditions (0°C
and 1013.3 mbars pressure). For this you
will need to measure the room tempera-
ture and the air pressure extant at the
time the experiment is being performed.
Fig. 1 Measuring the volume of the escaped air
