3B Scientific Boyle's Law Apparatus User Manual
Instruction sheet
3
Instruction sheet
3B SCIENTIFIC® PHYSICS
U17210 Boyle’s law apparatus
5/03 ALF
®
This apparatus is used for the experiment-based deter-
mination of the relationship between the volume and
the pressure of a gas (air) at constant temperature (Boyle’s
law).
1. Description, technical data
The apparatus consists of an enclosed plexi-glass cylin-
der with graduated scale to determine volume and a
flange-mounted manometer for pressure readings and
includes an aeration and de-aeration valve. By turning
the knob the threaded rod moves the piston up an down
inside the cylinder thus varying the volume. This permits
the generation of over- and underpressure. Two O-rings
attached to the piston seal off the air. These are lubri-
cated with a small amount of silicon oil. For safety rea-
sons the power cylinder is encased in an additional plexi-
glass cylinder.
Power cylinder:
Length:
300 mm
Diameter:
40 mm (interior)
Piston:
30 mm x 40 mm Ø
Scale:
Length:
250 mm
Scale div.:
5 mm
Manometer:
Pressure range:
–10 N/cm² - 30 N/cm²
Diameter:
100 mm
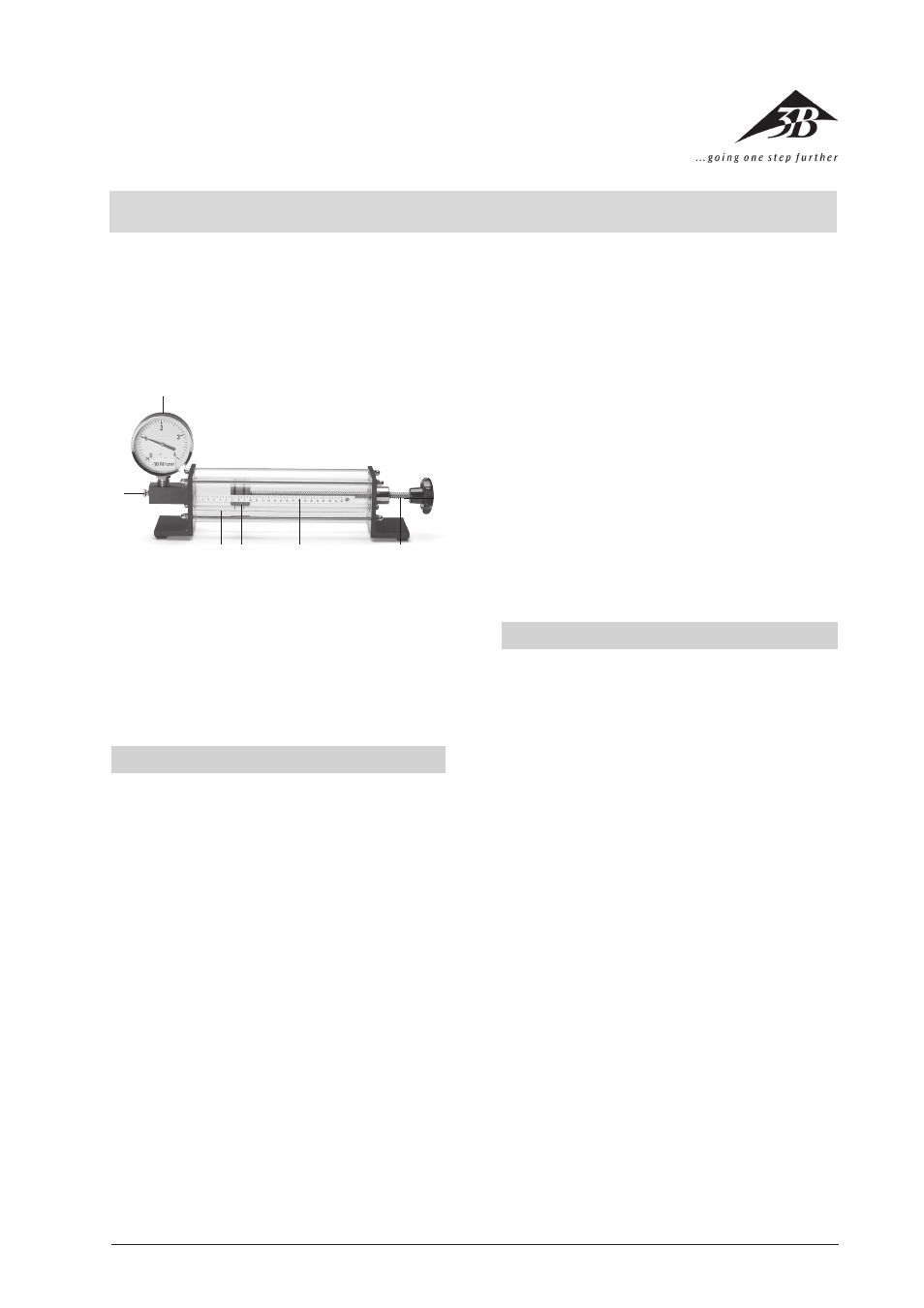
1
Manometer
2
Handscrew for the metering valve
3
Working cylinder with protective cylinder
4
Piston with O-rings
5
Scale
6
Rotary knob with threaded rod
1
2
4
3
5
2. Operation
Perform an experiment to verify Boyle’s law which states
that for a given mass of gas (air) at a constant tempera-
ture the product made up of the volume and the pres-
sure is constant.
The volume of the air column is computed out of the
product of the cylinder’s cross-section and the length of
the air column. As the cross-section is a fixed variable,
the change in volume can only be expressed by varying
the length of the air column.
• Ventilate the cylinder by turning the hand valve screw
to the left.
• Set the piston to the 25 cm mark. If the piston is stuck
the best remedy is to turn it slightly right to left, so
that the O-rings come into contact with the silicone
oil.
• Close the valve. The manometer gage pointed indi-
cates an initial pressure of 1.
• Before each pressure reading tap your finger softly
against the manometer to make sure that the pointer
is on the right setting.
• Turn the rotary knob to slide the piston to the 24 cm
mark and read off and note down the next pressure
level.
• Repeat the procedure in 1 cm steps.
• Enter all the values into a graph (see Figure).
• Proceed accordingly for the case that Boyle’s law is to
be verified for decreasing pressure. Start here with an
air column length of 7 cm.
6
