3B Scientific Daniell Cell User Manual
Page 2
4
Dimensions:
105 mm high, 65 mm Ø
Connections:
via 4-mm plugs
Appropriate filling:
Copper sulphate solution (CuSO
4
),
10%,
Zinc sulphate solution (ZnSO
4
), 10%
2.1 Scope of delivery
1
Glass vessel
1
Clay cylinder
1
Copper electrode with plug
1
Zinc electrode with plug
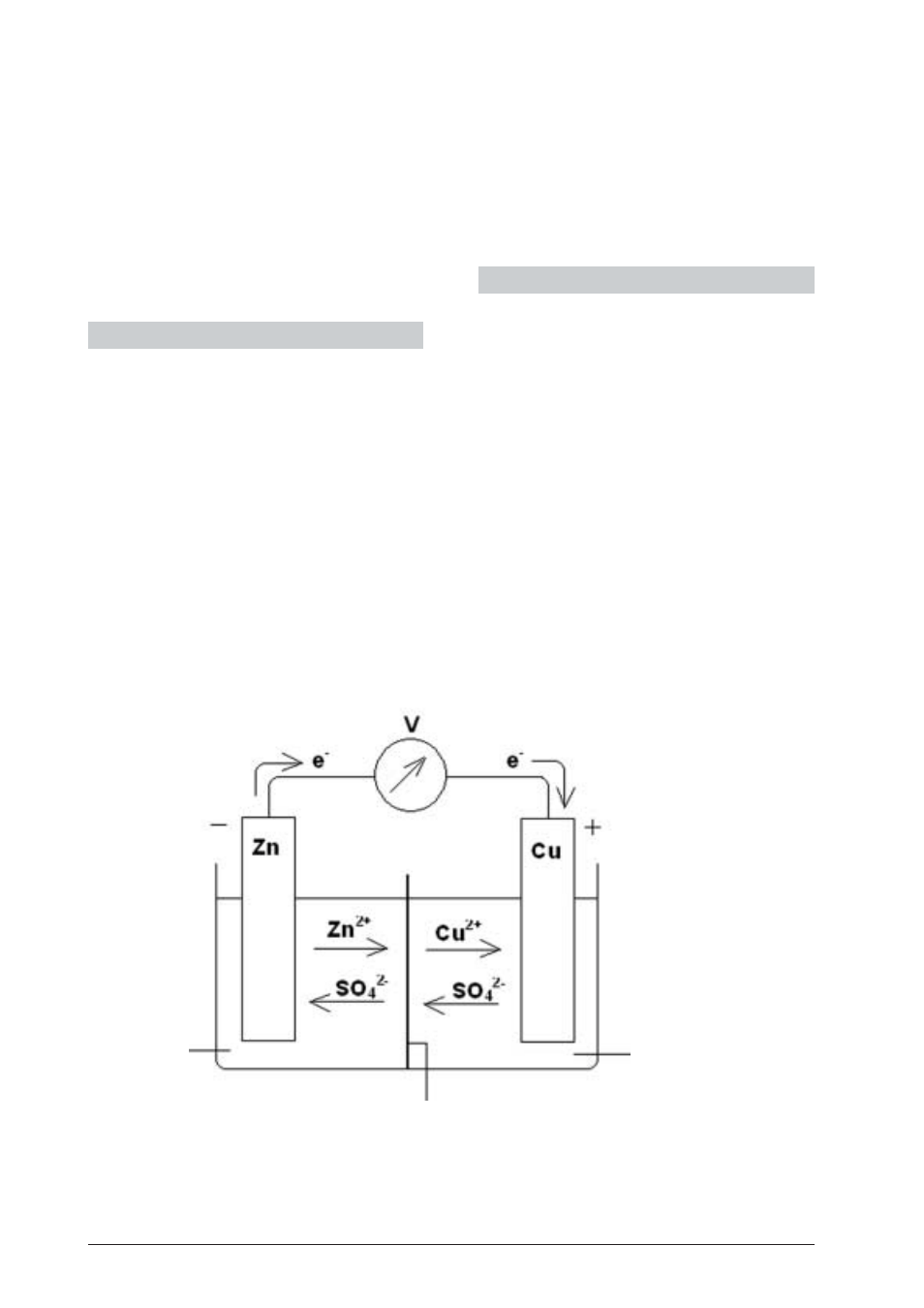
3. Theory
The combination of two half-cells for the purpose of
converting chemical energy into electrical energy is called
a galvanic cell. In a Daniell cell, a copper electrode with a
copper sulphate solution is situated in one half-cell and
a zinc electrode with a zinc sulphate solution is found in
the second. In galvanic cells, the baser metal of the two
always forms the negative pole. In this case, the elec-
trons thus flow from zinc to copper. The zinc electrode is
dissolved over a period of time, while metal copper set-
tles on the copper rod. The inner conduction is carried
out by the negative sulphate ions which can pass the
wall of the clay cylinder. Current drain is completed ei-
ther when the zinc electrode is dissolved or when the
copper sulphate solution has been used up. The follow-
ing reactions can be appreciated:
Oxidation:
−
→
+
2+
Zn
Zn
2e
Reduction:
−
+
→
2+
Cu
2e
Cu
Redox reaction:
→
+
2+
2+
Zn+ Cu
Zn
Cu
Theoretically, the Daniell cell provides a current of 1.1 V.
However, the measured reading, as a rule, is always slightly
below the theoretical value.
4. Operation
•
Before the start of the experiment, prepare a suffi-
cient amount of the electrolyte solutions.
•
For preparing one litre of 1-molar copper sulphate
solution, take 249.5 g CuSO
4
and add distilled water
till you get one litre of solution.
•
For preparing one litre of 1-molar zinc sulphate solu-
tion, take 287.4 g ZnSO
4
and add distilled water till
you get one litre of solution.
•
For preparing a 0.1-molar solution, only take 1/10
th
of
the specified quantities.
•
Pour the solutions into the corresponding half-cells.
•
Measure the current generated with a voltmeter.
•
The experiment can also be repeated with a 1-molar
copper sulphate and zinc sulphate solution.
•
The apparatus and electrodes must be thoroughly
cleaned immediately after the experiment.
•
Chemicals which cannot be reused must be stored in
special vessels and disposed of in an orderly fashion
afterwards, strictly adhering to current regulations.
Fig. 1: Daniell-Element
1
Zinc sulphate (ZnSO
4
)
solution
2
Porous partition
3
Copper sulphate (CuSO
4
)
solution
1
2
3
3B Scientific GmbH • Rudorffweg 8 • 21031 Hamburg • Germany • www.3bscientific.com • Technical specifications subject to change
