3B Scientific Leclanche Cell User Manual
Page 2
4
3B Scientific GmbH • Rudorffweg 8 • 21031 Hamburg • Germany • www.3bscientific.com • Technical specifications subject to change
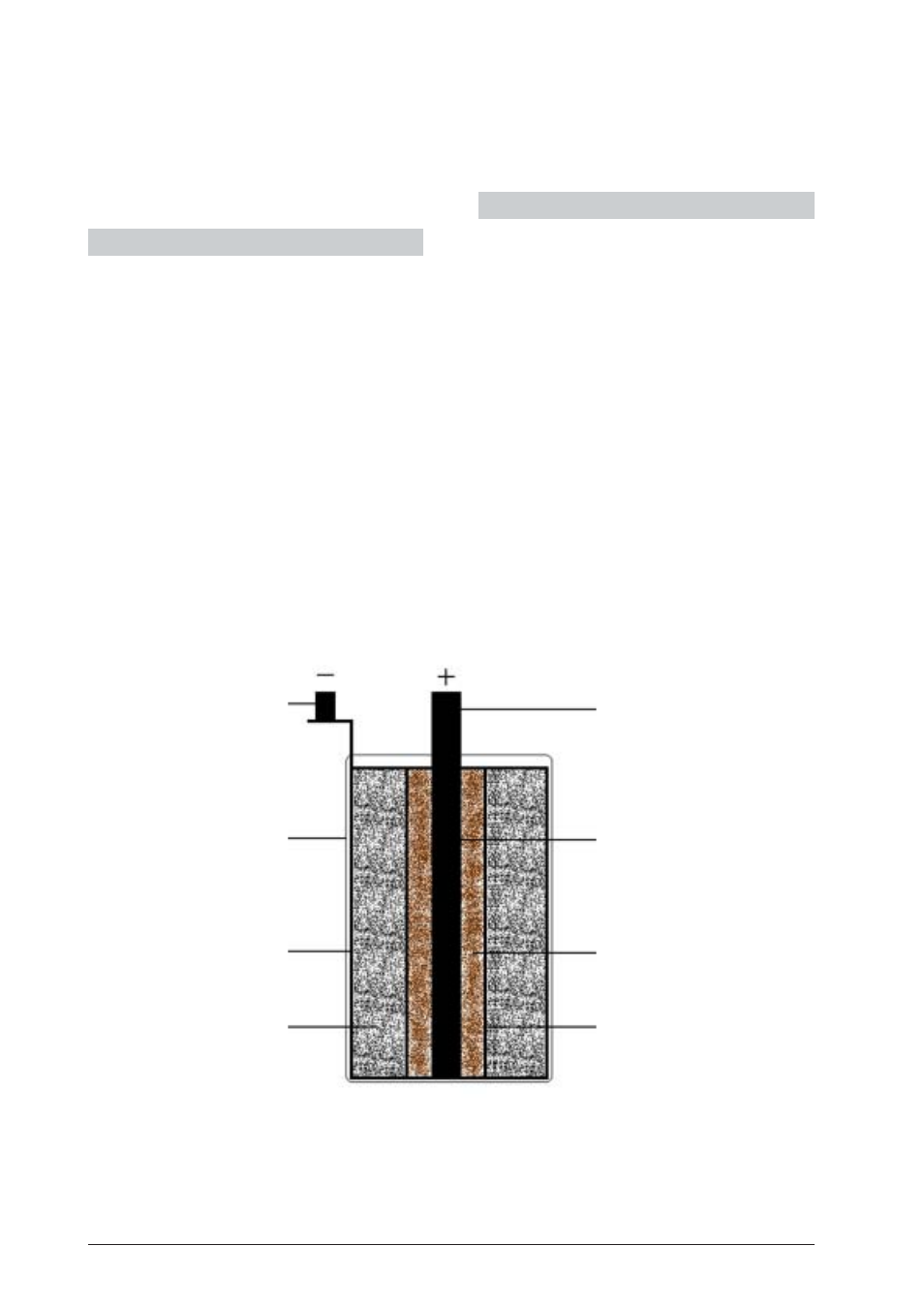
Fig. 1: Leclanché cell
1
Ceramic cylinder
2
Manganese dioxide coating
3
Carbon electrode
4
4-mm socket
5
Glass vessel
6
Zinc cylinder
7
Ammonium chloride
solution
4
4
5
6
7
1
2
3
2.1 Scope of delivery
1
Glass vessel
1
Clay cylinder
1
Lid
1
Zinc electrode with socket
1
Carbon electrode with socket
3. Principle
The combination of two half-cells for the purpose of
converting chemical energy into electrical energy is called
a galvanic cell. In a Leclanche cell, a zinc electrode forms
the negative pole and a carbon rod with the manganese
dioxide (MnO
2
) coating forms the positive pole. In the
space between, ammonium chloride is used as an elec-
trolyte. The ensuing chemical reaction chiefly results from
the oxidation of zinc and reduction of manganese diox-
ide.
Oxidation:
( )
+
−
+
→
+
+
2
+
4
3 2
Zn+ 2 NH
Zn NH
2e
2H
Reduction:
−
+
+
→
+
+
2
2
3
2
2 MnO
2 H
2 e
Mn O
H O
Redox reaction:
( )
+
+
→
+
+
2
+
4
2
3
2 3
2
2
Zn+ 2 NH
2 MnO
Zn NH
Mn O
H O
The reactions shown here are simplified. They are far
more complicated in reality. The reaction ceases when
the manganese dioxide has been used up.
4. Operation
•
To construct a Leclanché cell requires the following:
Ammonium chloride solution (NH
4
Cl), approx. 20%
Manganese dioxide (powder) (MnO
2
)
Graphite (powder)
•
Mix the manganese dioxide powder and some graph-
ite powder in a beaker. Then add the ammonium
chloride solution and stir the mixture to form a paste.
•
Position the zinc electrode into the glass vessel and
place the ceramic cylinder inside.
•
Position the carbon electrode in the centre of the
ceramic cylinder and fill up all remaining space with
the manganese dioxide paste.
•
Fill up the glass vessel with the 20% ammonium chlo-
ride solution and cover it with the lid.
•
The apparatus and electrodes must be thoroughly
cleaned immediately after the experiment.
•
Chemicals which cannot be reused must be stored in
special vessels and disposed of in an orderly fashion
afterwards, strictly adhering to applicable regulations.
