Technical data – Omron NE-C801 User Manual
Page 26
26
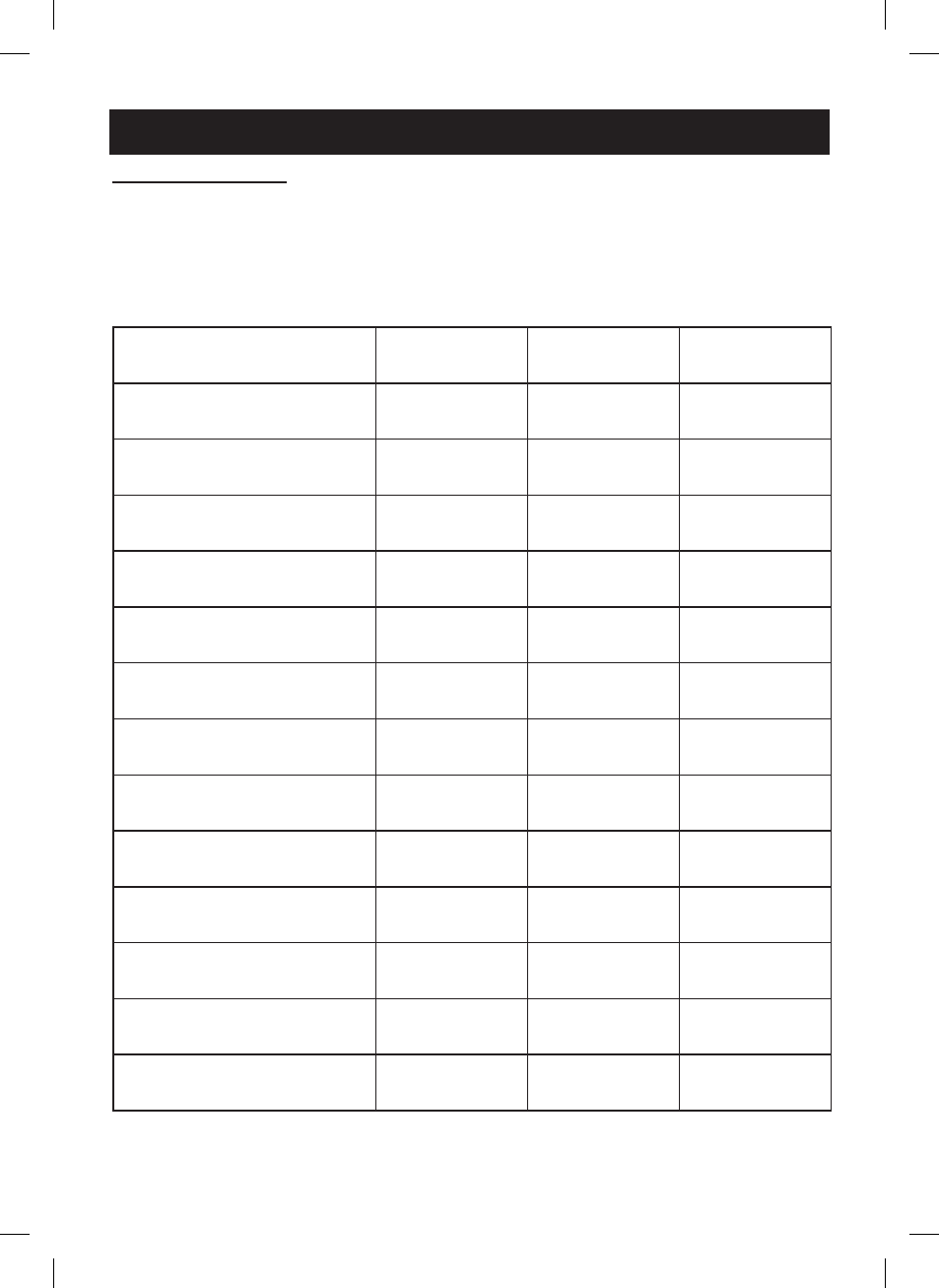
Particle Specifications
A series of aerosol performance tests were performed using an 8 stage cascade impactor at a
sampling flow rate of 15 l/min equipped with a USP <601> induction port throat. Aerosol was
sampled directly from the outlet. Three (3) device samples were tested with 3 runs each, for a total
of 9 sample points per each drug.
The specifications are listed below with intervals given for a 95% confidence level.
Mean / Std. Dev
Pulmicort
®
(250μg/mL)
Intal
®
(10mg/mL)
Salbutamol
®
(5mg/mL)
Total Delivered Dose (μg)
391.11 ± 16.51
12368.89 ± 269.61 7883.33 ± 116.96
Total Delivered Dose Fraction (%)
78.2% ± 3.3%
61.8% ± 3.1%
77.0% ± 2.5%
Particle size (MMAD) μm
3.88 ± 0.28
2.91 ± 0.14
2.54 ± 0.28
Geometric Standard Deviation
1.93 ± 0.20
2.27 ± 0.02
2.62 ± 0.03
Respirable Fraction (0.5-5μm)
61.9% ± 4.0%
70.5% ± 1.3%
66.4% ± 1.6%
Total Respirable Dose
(μg between 0.5-5μm)
242.17 ± 18.90
8729.49 ± 497.57
5235.02 ± 233.61
Medication Captured on USP Throat
13.34 ± 3.40
253.66 ± 27.42
144.94 ± 16.32
Medication Captured on USP
Throat Fraction (%)
3.4% ± 0.4%
2.0% ± 0.1%
1.8% ± 0.1%
Medication Retained in Device
96.11 ± 9.61
8000.00 ± 438.21
2072.22 ± 257.90
Medication Retained in Device
Fraction (%)
19.2% ± 1.9%
40% ± 2.2%
41.4% ± 5.2%
Coarse Particle Fraction (%) (>4.7μm)
37.1% ± 2.9%
27.7% ± 1.5%
26.6% ± 2.3%
Fine Particle Fraction (%) (<4.7μm)
59.6% ± 3.5%
70.2% ± 1.5%
71.6% ± 2.3%
Ultra-Fine Particle Fraction (%)
(<1.0μm)
3.5% ± 1.7%
8.9% ± 2.1%
16.9% ± 5.3%
Note: Course particles (oro-pharyngeal deposition) and ultra-fine particles (exhaled) are not likely
to deposit in the patient’s airway and thus provide limited clinical benefit.
Technical DaTa
NE-C801S-Z_IM_E_SP_r3.indd 26
9/20/11 12:16:37 PM
