Merit Medical Sequitor Steerable Guidewire User Manual
Page 4
•
To aid in rotating or steering the guidewire,
secure the supplied torque device to the
proximal end of the guidewire.
•
In order to aid in the selective placement of
the catheter into a particular vessel, gently
rotate the proximal end of the guidewire as it
is advanced forward.
•
Use accepted angiographic techniques to
steer the guidewire to the intended location.
Warning: Always maintain visualization of the
guidewire under fluoroscopy, ensuring that the
tip is moving freely when torque is applied.
•
When the desired guidewire position is
achieved, secure the guidewire in place
while tracking the catheter over it and to the
treatment location.
•
Once the micro-catheter is in position, gently
remove the guidewire prior to any intervention.
CAUTION:
Federal (USA) law restricts this device to use by or
on the order of a licensed physician.
CONTRAINDICATIONS:
There are no known contraindications for the use of
steerable guidewires.
STORAGE:
Store the Sequitor Steerable Guidewire in a cool,
dark, dry area.
COMPATIBILITY:
The Sequitor Steerable Guidewire is compatible with
catheters which use 0.014” or 0.018” guidewires in
intravascular procedures.
WARRANTY
BioSphere Medical
®
warrants that reasonable care
has been used in the design and manufacture of this
instrument. This warranty is in lieu of and excludes
all other warranties not expressly set forth herein,
whether ex¬pressed or implied by operation of
law or otherwise, including, but not limited to, any
implied warranties of merchantability or fitness.
Handling, storage, cleaning and sterilization of this
instrument, as well as factors relating to the patient,
diagnosis, treatment, surgical procedures, and other
matters beyond BioSphere Medical’s control, directly
affect the instrument and the results obtained from
its use. BioSphere Medical’s obligation under this
warranty is limited to the repair or replacement of
this instrument. BioSphere Medical is not liable for
any incidental or consequential loss, damage, or
expense directly or indirectly arising from the use of
this instrument. BioSphere Medical neither assumes
nor authorizes any other person to assume for it
any other or additional liability or responsibility in
connection with this instrument.
BioSphere Medical assumes no liability with respect
to instruments reused, reprocessed or re-sterilized,
and makes no warranties, expressed or implied,
including, but not limited to, merchantability or
fitness for intended use with respect to such
instrument.
All serious or life threatening adverse events or
deaths associated with use of Sequitor should be
reported to the Competent Authority of the country
where it occurred and to the device manufacturer.
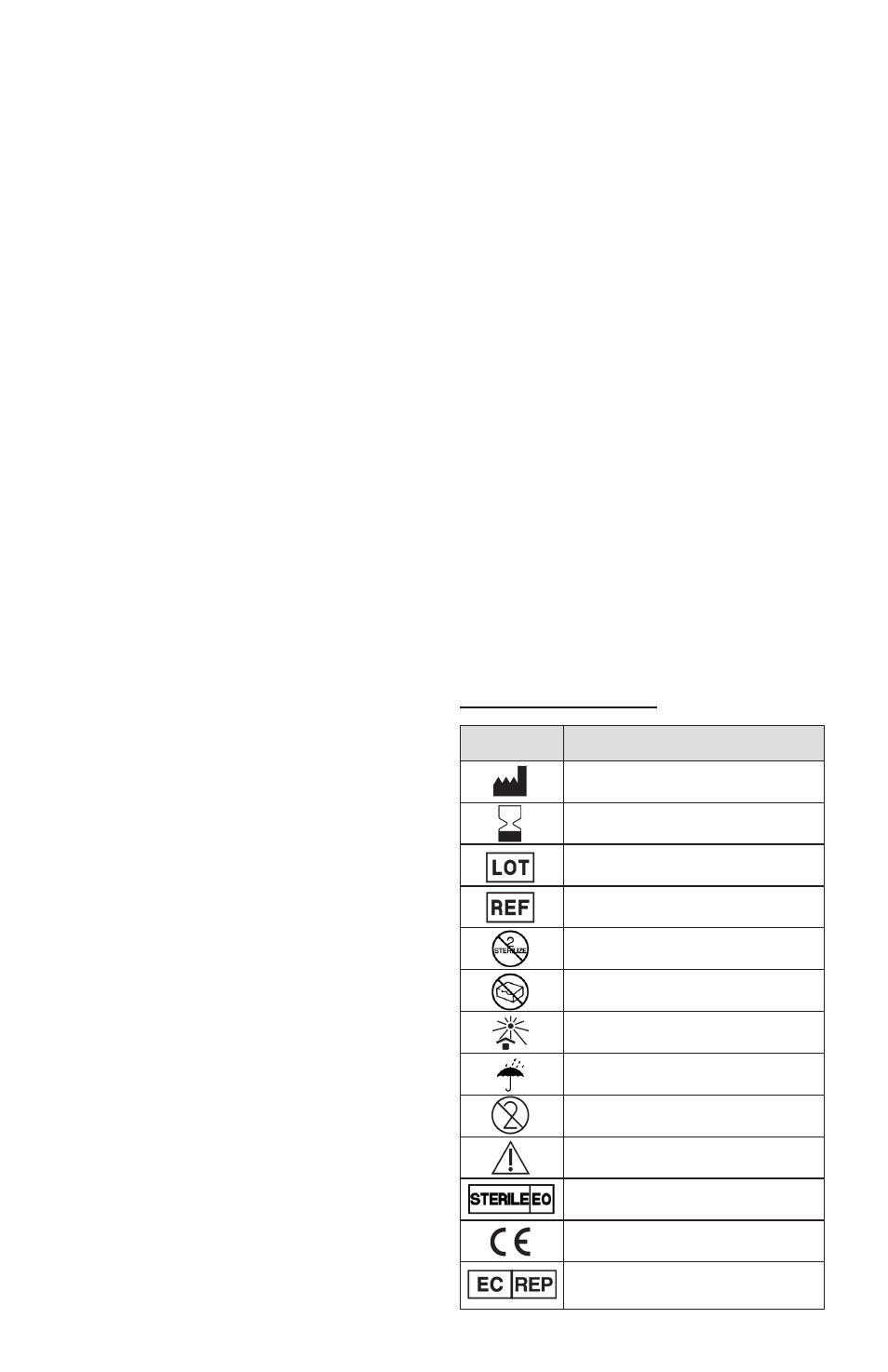
Information on packaging:
Symbol
Designation
Manufacturer: Name & Address
Use by date: year-month
Batch code
Catalogue number
Do not resterilize
Do not use if package is damaged
Keep away from sunlight
Keep dry
Do not re-use
Caution -
Refer to Instructions For Use
Sterilized using ethylene oxide
EC mark logo -
Notified body identification : 0459
Authorized Representative in
European Community
3
